Combustion Fundamentals
1. Chemical Kinetics
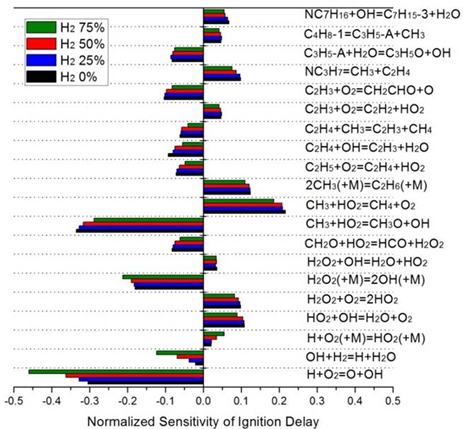
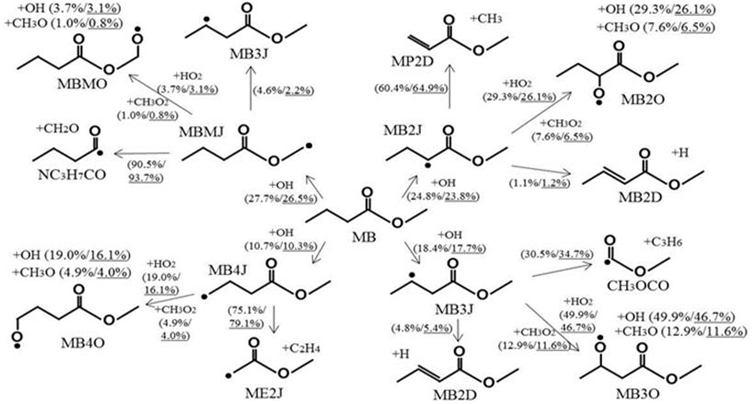
Chemical kinetics is one of the most important factors in the analysis of combustion reactions in terms of element reaction and its reaction rate during chemical reaction. It is the most important part of the combustion basic research to understand the reaction path of the combustion process because all fuels decompose by chemical reaction and produce energy. Through this research, it is possible to analyze in detail the reaction pathway from the change in combustion conditions such as fuel type, temperature, pressure, etc. We conduct research on this matter through experiments and numerical analysis.
2. Aerosol Measurement
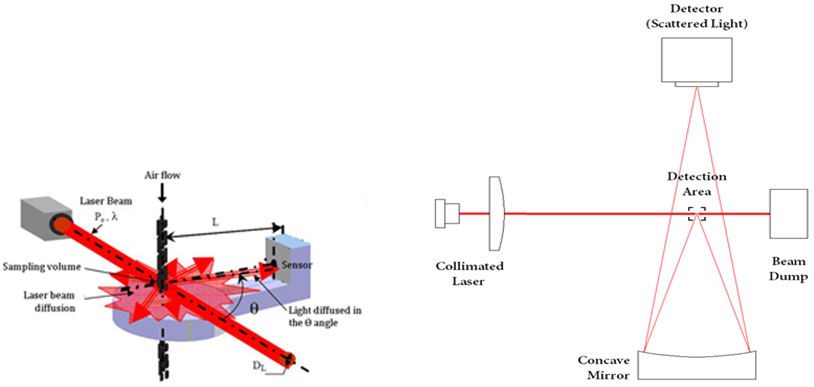
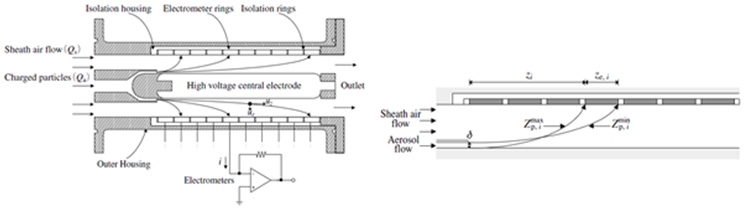
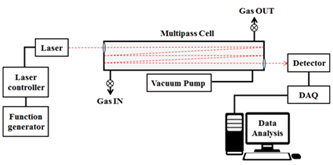
Combustion products can be divided into particles discharged in solid or liquid state and chemical species discharged in the state of gas, depending on their properties. Particulate substances can be measured in various ways depending on their components, properties and size. When measuring large particles of several hundred nm or more, a method of measuring the scattered light by irradiating a laser is mainly used and smaller particles can be measured by charging and then sorting using an electric field. The composition and concentration of the gaseous combustion products can be determined by measuring the wavelength band and intensity that are absorbed or transmitted by irradiating light of a specific wavelength. Studies are being carried out to analyze the combustion process and accurate combustion analysis at the level of chemical kinetics by measuring the composition and concentration of various combustion products.
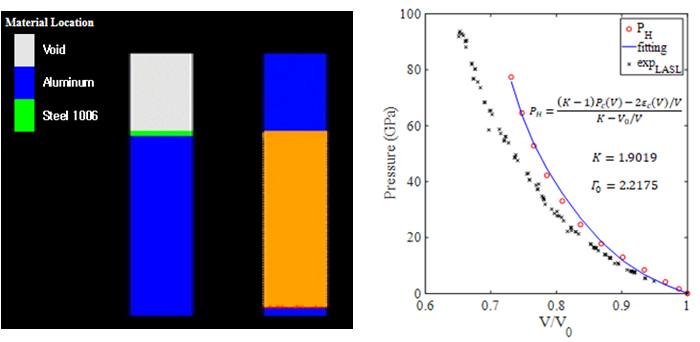
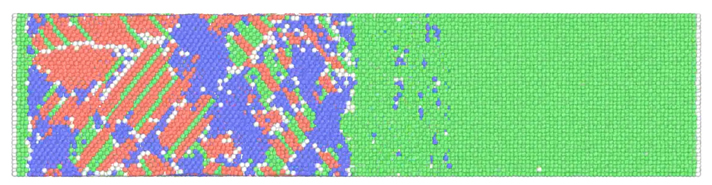
3. Energetic Material
Multifunctional energetic materials are contradictory substances that are normally used as a stable structure and release energy rapidly when necessary. In this way, in order to find substances that simultaneously satisfy structural stability and fast energy release characteristics, it is approached with a multiscale numerical analysis method. The multiscale numerical analysis method is a study to derive the thermodynamic and physical properties of a substance by analyzing it by connecting the atom scale of nm unit, mesoscale of μm unit and continuous scale of mm unit.